INTRODUCTION
Students of chemistry and materials normally have to learn the full periodic table (Appendix I) and the detailed electron configuration (Appendix II) in the first year of their college life. The sad fact is that this is something they do not naturally like: at first sight, they are just a bunch of numbers mixed with letters. Remembering the sequence and the associated number of electrons is certainly an unimaginable task. My way of teaching it is to create a "wow" effect first: I ask the class to call out any atomic number (or, better still, the element), then I sound out in music while writing down the electron configuration for that element. "Wow" the students exclaim every time without fail. The interest is thus planted and the desire to find out how to do it is aroused.
THE MUSICAL WAY
First, define the musical note in seven digits. This is extremely simple. Students from a Chinese background do not have to learn this, because they have all learned the "simplified musical note" in primary school. For students of other origins, a definition (Fig. 1(a)) may be necessary: view these "digitized" musical notes ("do, re, me, fa, so, la, ti" for 1 through 7) as the primary quantum numbers (the primary shells). Writing down the primary quantum numbers following the Aufbau principle makes a nice piece of music:
1223 343 4545 645 6756 7·····························································(1)
Or "do re re me—me fa me—fa so fa so—la fa so—la ti so la ti", as given in Fig. 1(b). This is step 2.
In step 3, assign an appropriate orbital letter (s, p, d or f subshell) to each of the "musical notes". For this purpose, first identify the same shell numbers, noting that they may not be together—as shown in expression (1) for the bold "5" s. It is important to show an example at this stage. For instance, assign orbitals to the "5" s: affix the s-orbital to the first "5", p to the second "5", d to the third "5" and "f" to the fourth "5". As all the "5" s are assigned to the orbitals, expression (1) becomes
1223 343 45s45p 645d 675f6 7·······················································(2)
It is necessary to stress to the student that not all of the shell numbers will use up the orbital letters. For shell number 1, only s will be used. For shell number 2, only s and p are used; the d and f orbitals are not used. When all the shell numbers are assigned orbital letters, expression (1) becomes
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p·····················(3)
This is Aufbau's building up principle written on one line. Expression (3) is the "master skeleton" for electron configuration of all the elements in the periodic table.
Step 4, or the final step, is to fill up the electrons in each orbital. It is now time to get the student to focus on the maximum number of electrons each orbital can accommodate: s up to 2; p up to 6; d up to 10; and f up to 14 (as tabulated in Table 1).
Drawing an analogy with a hotel (beds, rooms and floors) always work wonders in explaining the number of electrons the orbital can "house". Also, students appreciate having pointed out to them the "plus 4" rule: 2 plus 4 is 6 (max for p), 6 plus 4 is 10 (max for d); and 10 plus 4 is 14 (max for f).
At this point of time, a quiz is a good idea, to allow the student to digest the information and put the "music way" to work. For instance, complete the electron configuration of 32Ge. The answer should be 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p².
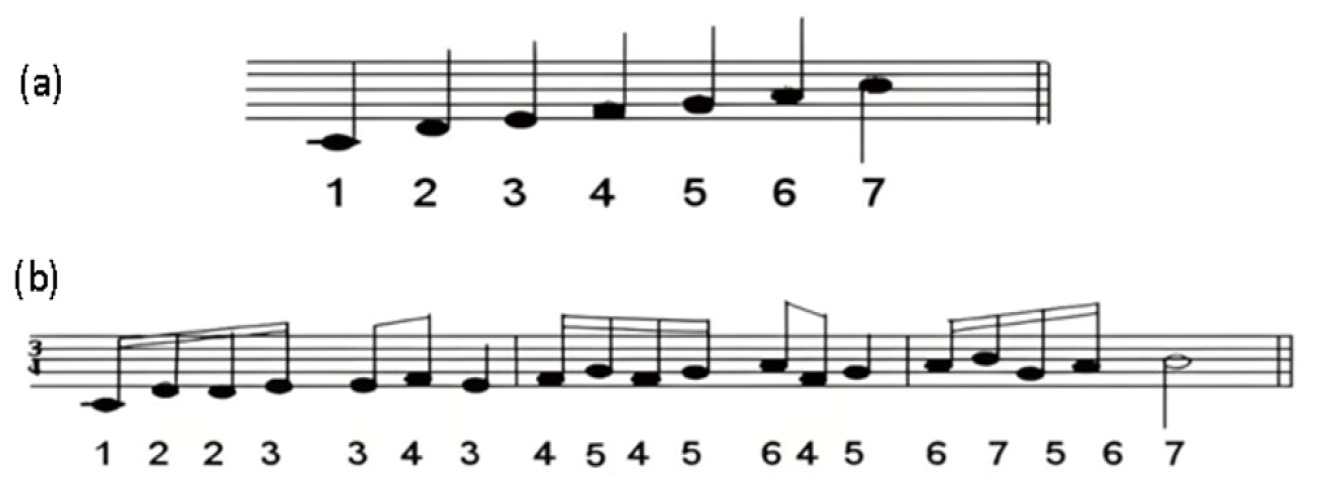
Fig.1. The musical way: (a) digitizing the basic musical note: ‘do re me fa so la ti’ as numbers 1 through 7, (b) electron shell arrangements in music ‘do re re me—me fa me—fa so fa so—la fa so—la ti so la ti’.
Table 1 Maximum number of electrons the orbitals can accommodate

Finally, it is necessary to note the few exceptions in filling up the orbital: in some elements, the electrons do not completely fill up the second last orbitals before moving on to fill up the outermost orbital. These elements are Cr, Cu, Nb, Pd, Ce, Tb, Pa and Bk. For instance: 24Cr: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵ or 29Cu: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹3d¹⁰. The irregularity of the number of the electrons is high-lighted in bold. These exceptions do not affect the "music way" nor the assignment of the orbital letters.
SUMMARY
The electron configuration of elements can be taught in a musical way:
Step 1: "Digitize" the basic musical notes "do re me fa so la ti" as numbers 1 through 7
Step 2: Sound out in music the primary shell numbers in the Aufbau building up principle: 1223 343 4545 645 6756 7 or "do re re me—me fa me—fa so fa so—la fa so—la ti so la ti"
Step 3: Assign appropriate s, p, d, f orbitals to each number: 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p
Step 4: Fill up the orbitals with the number of electrons as superscripts to the orbital letter up to their respective maximum: s up to 2; p up to 6; d up to 10; and f up to 14. Note the few exceptional elements (Cr, Cu, Nb, Pd, Ce, Tb, Pa and Bk) that do not completely fill up the second last orbitals before moving on to fill up the outermost orbitals. All the superscripts must add up to equal the atomic number.
APPENDIX
1. THE MODERN PERIODIC TABLE: A REVIEW
The arrangement of elements in the modern periodic table is based on the electron configuration of atoms. Electron configuration refers to the order in which electrons are arranged around the nucleus of an atom.
The periodic table contains rows and columns. The rows are called periods. The columns are called groups. There are 7 periods and 18 groups in the modern periodic table (Fig. 2).
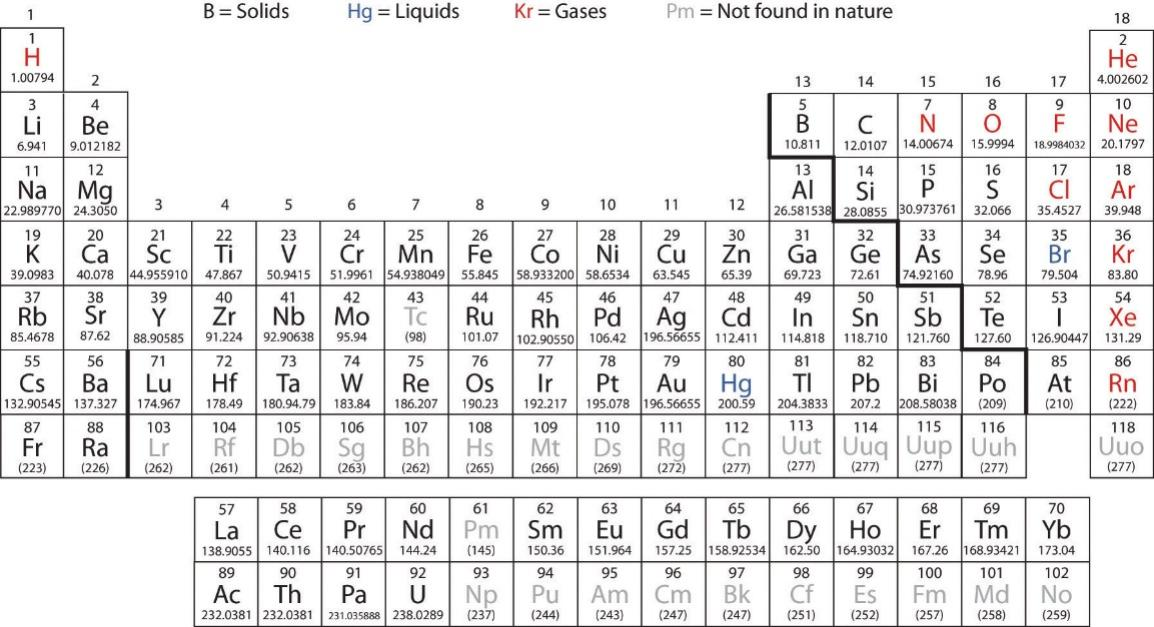
Fig. 2. The modern period table of elements.
The periods represent the energy levels indicated by the principal quantum numbers 1, 2, 3, 4, 5, 6 and 7. The 18 groups of elements have been classified according to the orbitals (s, p, d and f) because the electrons in these orbitals determine their chemistry. The s-orbital accommodates up to 2 electrons spinning in opposite directions, the p-orbitals up to 6 electrons (3 p-orbitals each can "house" 2 electrons spinning in opposite directions); the d-orbital up to 10 electrons (5 d-orbitals times 2 each) and the f-orbital 14 electrons (7 orbitals times 2 each)
2. THE ELECTRON CONFIGURATION
As electrons fill up the orbitals in an atom, they do so in an effort to minimize the total energy. The energy of an electron depends on the combination of its primary quantum number and the angular momentum; thus 4s is filled up before 3d, etc. Filling of the orbitals follows Aufbau"s principle (Fig. 3): the sequence in which the orbitals are filled represents the increasing energy of these orbitals. Spatially, the orbital with the highest principal quantum number is the furthest from the nucleus. The number of electrons of an elemental atom equals the atomic number; thus, given the atomic number of an element, the electron configuration can be written according to Aufbau's building up principle. For instance, Germanium has an atomic number of 32 (i.e. it has 32 electrons), thus the electron configuration of 32Ge is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p².
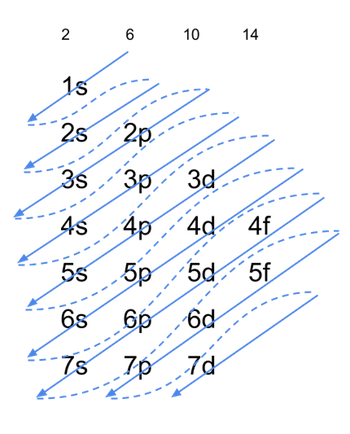
Fig. 3. Aufbau's Building Up Principle
